The metallothermic SHS of cast CoCrFeNiMn alloys in conditions of artificial gravity was described in detail in [32].
SHS-produced cast alloys were characterized by X-ray diffraction (XRD) analysis, scanning electron microscopy (SEM), and energy dispersive microanalysis (EDS). In order to reveal nanostructured constituents, Al-containing alloys were subjected to etching in a 5% nitric acid solution followed by neutralization of solution.
The SHS reaction yielding NiCrCoFeMn–(X) alloys can be represented by the following scheme:
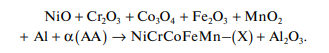
where AA is an alloying additive (Al and Ti–Si– B(C)), whose concentration was varied within the range 0.2–1.0 mole fraction for Al and 1–8 wt % for Ti–Si–B(C). The main components were used in equal atomic fractions.
The authors of [30–33] previously noted that the action of gravity forces favors the separation of combustion product into two layers (target product ingot and slag Al2O3) and a convective mixing of all components, which is especially important with an increase in the number and concentration of components in the alloy. Therefore, the synthesis of HEAs was carried out in a centrifugal SHS machine [30].
RESULTS AND DISCUSSION Synthesis of Cast NiCrCoFeMn–Al HEAs
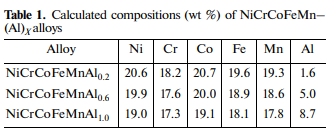
The introduction of aluminum in excess of stoichiometry into the exothermic green mixture makes it easy to control its concentration in the composition of produced alloy; therefore, this method of alloying of the initial HEA was applied. Because of the low specific density of Al, an increase in its concentration promotes a decrease in the specific density of the alloy, as well as with allowance made for the high reactivity and formation of aluminides, contributes to strengthening. The compositions of synthesized HEAs are given in Table 1. In order to determine the optimal conditions for the preparation of alloys, we carried out experiments on the variation of a (centrifugal acceleration) between 1 and 70 g. Our experiments showed that, with increasing a, the burning velocity (Ub) increases from 2 to 6.1 cm/s for the NiCrCoFeMnAl0.2 composition and from 2 to 4.6 cm/s for the NiCrCoFeMnAl1.0 composition.
Note that the increase in Ub is the greatest between 10 and 50 g. This occurs due to forced filtration of a high-temperature melt formed behind the combustion front into the green mixture [30]. An additional point to emphasize is that, as g grows in parallel with the increase in Ub, the loss of material markedly decreases and the yield of target material into ingot approaches the calculated value.
The ingots prepared at a/g ≤ 50 were porous (gas inclusions). At a/g ≥ 50, the samples became pore-free and their mass was close to nominal one (~98 wt %). In this case, the material splashing during combustion did not exceed 1.5 wt %. Synthesized products were obtained as two-layer samples: target alloy and Al2O3 (slag). The ingots formed in optimal conditions had no residual porosity and were monolithic.
As a result, the values of a > 50 g were chosen as optimal. EDS analysis revealed no change in the concentrations of components over the entire bulk. The insignificant deviations in their values are within the measured error range. It is important to note that the contents of components are slightly lower than the nominal values (less than 2%), with the exception of Mn (6%). The difference was eliminated by introducing manganese oxide (MnO2) in excess of stoichiometry into the green composition.
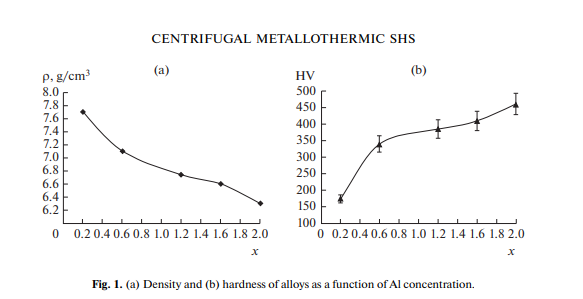
An analysis of samples optimized in the composition showed that an increase in the Al concentration in the alloy leads to a noticeable decrease in the density of synthesized alloys (Fig. 1a); in this case, there is a significant increase (of more than 2 times) in their hardness (Fig. 1b). The marked growth is observed within the range X = 0.2–0.6.
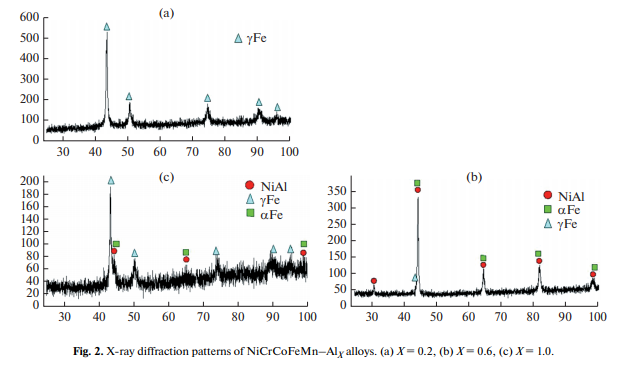
The latter can be explained by the formation of “solid” inclusions of intermetallic phases based on aluminides. An XRD analysis of cast HEAs prepared at a = 55 ± 5 g showed the dependence of phase composition on the Al concentration (Fig. 2). At X = 0.2, a single-phase product with fcc structure is formed. For X = 0.6–1.0, the combustion products are seen to consist of an α-Fe (bcc) phase, a γ-Fe (fcc) phase, and an intermetallic β-NiAl phase.